

圓形微腔設計太大使得溶液浪費且測試過程中基底的更換、池體(ti) 的密封困難。然後完成圓環型微元腔體(ti) 的設計及製作,其足夠小的腔體(ti) 減小了溶液對橢偏測試帶來的影響。但是容納的溶液會(hui) 帶來沉積離子不夠的問題且經過實驗發現對電極ITO上會(hui) 出現氣泡,影響橢偏測試,所以在此基礎上設計製作完成了長方形微流腔體(ti) 。
展示全部 
橢偏儀(yi) 在位表征電化學沉積的係統搭建(二十)- 長方形流動微腔
3.3.2長方形流動微腔
為(wei) 了解決(jue) 溶液微圓形腔體(ti) 溶液注入困難、溶液少及反應產(chan) 生氣泡的問題,進一步對腔體(ti) 進行改進,得到微流腔體(ti) 。圖3-18是微流腔體(ti) 製作過程示意圖,首先準備好ITO、EVA膠膜、特氟龍細管、Au/Si,把EVA膠膜切成內(nei) 為(wei) 25px×62.5px,外為(wei) 37.5px×75px的長方形,然後從(cong) 上到下依次把ITO、EVA膠膜/特氟龍細管、Au/Si疊好置於(yu) 加熱平台,在150℃下加熱,使得EVA軟化粘合池體(ti) ,zui後冷卻得到成品。
該池體(ti) 工作電極即為(wei) Au/Si基底,上端的ITO即為(wei) 對電極,溶液的進出由兩(liang) 邊的特氟龍細管實現,通常製作完成後的微腔厚度和特氟龍細管一致,約為(wei) 1mm。該微腔具有液層薄、溶化充足且流動可以消除ITO上產(chan) 生的氣泡等優(you) 點。
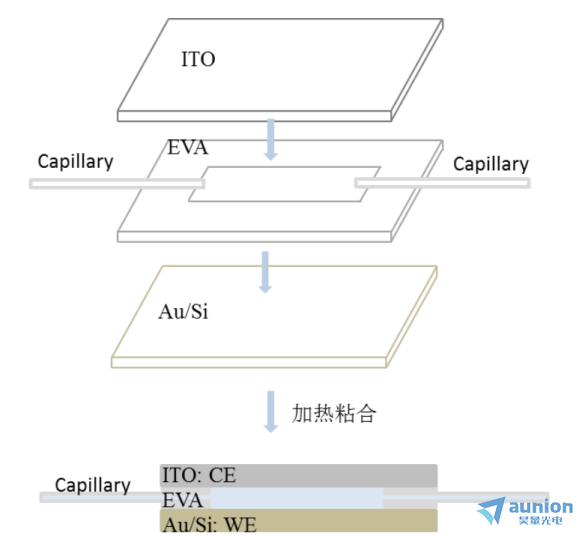
圖31-18流動型微腔示意圖
用該池體(ti) 對不同濃度醋酸鉛溶液進行測試,得到的結果如圖3-19所示。可以看到有無溶液加入,測試得到的橢偏參數峰位及數值上都存在差別。但是在加不同濃度的溶液(去離子水、1M醋酸鈉、1M的醋酸鈉和5/10/15/20mM的醋酸鉛)後得到的橢偏參數數值和趨勢都一致。這和前麵所述的半圓弧型電解池在不同濃度的醋酸鉛溶液中橢偏測試結果一致,同樣說明在醋酸鉛溶液中,其濃度橢偏測試參數的影響可忽略不計。
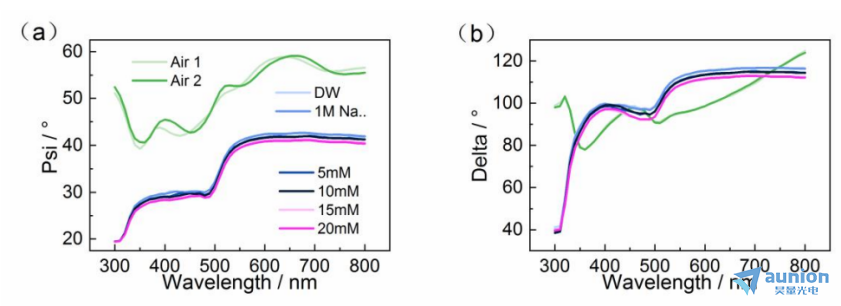
圖3-19不同條件下EVA腔體(ti) 橢偏測試結果(a)Psi;(b)Delta
如圖3-20所示,是用該池體(ti) 進行沉積薄膜的結果。電解液為(wei) 0.02MCu(CH3COO)2,0.1MCH3COONa,Au/Si為(wei) 工作電極,ITO為(wei) 對電極,-0.4mA恒壓沉積。對比沉積前後腔體(ti) 圖可知,用該池體(ti) 可以進行沉積。與(yu) 前圓環電極對比可以看到,ITO上不再有氣泡存在,因為(wei) 產(chan) 生的氣泡都被流動的溶液帶走了。因溶液可以流動,故可克服圓環電極溶液少的缺點。所以後續沉積薄膜實驗的橢偏儀(yi) 監測選用該流動池體(ti) 進行。
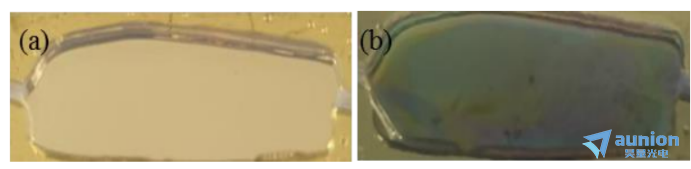
圖3-20流動型微腔(a)沉積前(b)沉積後實物圖
3.4小結
本文主要介紹了研究中實驗裝置的設計及測試的過程,主要包含半圓弧型器件微元腔體(ti) 器件。首先設計完成半圓弧器件,實現了把沉積過程和橢偏儀(yi) 測試相結合,觀察窗口選用石英玻璃,理論上zui大限度減小了光的損耗。但是它的池體(ti) 設計太大使得溶液浪費且測試過程中基底的更換、池體(ti) 的密封困難。然後完成圓環型微元腔體(ti) 的設計及製作,其足夠小的腔體(ti) 減小了溶液對橢偏測試帶來的影響。但是容納的溶液會(hui) 帶來沉積離子不夠的問題且經過實驗發現對電極ITO上會(hui) 出現氣泡,影響橢偏測試,所以在此基礎上設計製作完成了長方形微流腔體(ti) 。該設計成功解決(jue) 了氣泡和溶液少所帶來的問題,故而後續實驗將采用長方形微流腔體(ti) 。
了解更多橢偏儀(yi) 詳情,請訪問上海昊量光電的官方網頁:
https://www.weilancj.com/three-level-56.html
更多詳情請聯係昊量光電/歡迎直接聯係昊量光電
關(guan) 於(yu) 昊量光電:
上海昊量光電設備有限国产黄色在线观看是光電国产欧美在线專(zhuan) 業(ye) 代理商,国产欧美在线包括各類激光器、光電調製器、光學測量設備、光學元件等,涉及国产成人在线观看免费网站涵蓋了材料加工、光通訊、生物醫療、科學研究、國防、量子光學、生物顯微、物聯傳(chuan) 感、激光製造等;可為(wei) 客戶提供完整的設備安裝,培訓,硬件開發,軟件開發,係統集成等服務。
您可以通過我們(men) 昊量光電的官方網站www.weilancj.com了解更多的国产欧美在线信息,或直接來電谘詢4006-888-532。
相關(guan) 文獻:
[1] WONG H S P, FRANK D J, SOLOMON P M et al. Nanoscale cmos[J]. Proceedings of the IEEE, 1999, 87(4): 537-570.
[2] LOSURDO M, HINGERL K. ellipsometry at the nanoscale[M]. Springer Heidelberg New York Dordrecht London. 2013.
[3] DYRE J C. Universal low-temperature ac conductivity of macroscopically disordered nonmetals[J]. Physical Review B, 1993, 48(17): 12511-12526. DOI:10.1103/PhysRevB.48.12511.
[4] CHEN S, KÜHNE P, STANISHEV V et al. On the anomalous optical conductivity dISPersion of electrically conducting polymers: Ultra-wide spectral range ellipsometry combined with a Drude-Lorentz model[J]. Journal of Materials Chemistry C, 2019, 7(15): 4350-4362.
[5] 陳籃,周岩. 膜厚度測量的橢偏儀(yi) 法原理分析[J]. 大學物理實驗, 1999, 12(3): 10-13.
[6] ZAPIEN J A, COLLINS R W, MESSIER R. Multichannel ellipsometer for real time spectroscopy of thin film deposition from 1.5 to 6.5 eV[J]. Review of Scientific Instruments, 2000, 71(9): 3451-3460.
[7] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[8] DULTSEV F N, KOLOSOVSKY E A. Application of ellipsometry to control the plasmachemical synthesis of thin TiONx layers[J]. Advances in Condensed Matter Physics, 2015, 2015: 1-8.
[9] YUAN M, YUAN L, HU Z et al. In Situ Spectroscopic Ellipsometry for Thermochromic CsPbI3 Phase Evolution Portfolio[J]. Journal of Physical Chemistry C, 2020, 124(14): 8008-8014.
[10] 焦楊景.橢偏儀(yi) 在位表征電化學沉積的係統搭建.雲(yun) 南大學說是論文,2022.
[11] CANEPA M, MAIDECCHI G, TOCCAFONDI C et al. Spectroscopic ellipsometry of self assembLED monolayers: Interface effects. the case of phenyl selenide SAMs on gold[J]. Physical Chemistry Chemical Physics, 2013, 15(27): 11559-11565. DOI:10.1039/c3cp51304a.
[12] FUJIWARA H, KONDO M, MATSUDA A. Interface-layer formation in microcrystalline Si:H growth on ZnO substrates studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Journal of Applied Physics, 2003, 93(5): 2400-2409.
[13] FUJIWARA H, TOYOSHIMA Y, KONDO M et al. Interface-layer formation mechanism in (formula presented) thin-film growth studied by real-time spectroscopic ellipsometry and infrared spectroscopy[J]. Physical Review B - Condensed Matter and Materials Physics, 1999, 60(19): 13598-13604.
[14] LEE W K, KO J S. Kinetic model for the simulation of hen egg white lysozyme adsorption at solid/water interface[J]. Korean Journal of Chemical Engineering, 2003, 20(3): 549-553.
[15] STAMATAKI K, PAPADAKIS V, EVEREST M A et al. Monitoring adsorption and sedimentation using evanescent-wave cavity ringdown ellipsometry[J]. Applied Optics, 2013, 52(5): 1086-1093.
[16] VIEGAS D, FERNANDES E, QUEIRÓS R et al. Adapting Bobbert-Vlieger model to spectroscopic ellipsometry of gold nanoparticles with bio-organic shells[J]. Biomedical Optics Express, 2017, 8(8): 3538.
[17] ARWIN H. Application of ellipsometry techniques to biological materials[J]. Thin Solid Films, 2011, 519(9): 2589-2592.
[18] ZIMMER A, VEYS-RENAUX D, BROCH L et al. In situ spectroelectrochemical ellipsometry using super continuum white laser: Study of the anodization of magnesium alloy [J]. Journal of Vacuum Science & Technology B, 2019, 37(6): 062911.
[19] ZANGOOIE S, BJORKLUND R, ARWIN H. Water Interaction with Thermally Oxidized Porous Silicon Layers[J]. Journal of The Electrochemical Society, 1997, 144(11): 4027-4035.
[20] KYUNG Y B, LEE S, OH H et al. Determination of the optical functions of various liquids by rotating compensator multichannel spectroscopic ellipsometry[J]. Bulletin of the Korean Chemical Society, 2005, 26(6): 947-951.
[21] OGIEGLO W, VAN DER WERF H, TEMPELMAN K et al. Erratum to ― n-Hexane induced swelling of thin PDMS films under non-equilibrium nanofiltration permeation conditions, resolved by spectroscopic ellipsometry‖ [J. Membr. Sci. 431 (2013), 233-243][J]. Journal of Membrane Science, 2013, 437: 312..
[22] BROCH L, JOHANN L, STEIN N et al. Real time in situ ellipsometric and gravimetric monitoring for electrochemistry experiments[J]. Review of Scientific Instruments, 2007, 78(6).
[23] BISIO F, PRATO M, BARBORINI E et al. Interaction of alkanethiols with nanoporous cluster-assembled Au films[J]. Langmuir, 2011, 27(13): 8371-8376.
[24] 李廣立. 氧化亞(ya) 銅薄膜的製備及其光電性能研究[D]. 西南交通大學, 2016.
[25] 董金礦. 氧化亞(ya) 銅薄膜的製備及其光催化性能的研究[D]. 安徽建築大學, 2014.
[26] 張楨. 氧化亞(ya) 銅薄膜的電化學製備及其光催化和光電性能的研究[D]. 上海交通大學材料科 學與(yu) 工程學院, 2013.
[27] DISSERTATION M. Cellulose Derivative and Lanthanide Complex Thin Film Cellulose Derivative and Lanthanide Complex Thin Film[J]. 2017.
[28] NIE J, YU X, HU D et al. Preparation and Properties of Cu2O/TiO2 heterojunction Nanocomposite for Rhodamine B Degradation under visible light[J]. ChemistrySelect, 2020, 5(27): 8118-8128.
[29] STRASSER P, GLIECH M, KUEHL S et al. Electrochemical processes on solid shaped nanoparticles with defined facets[J]. Chemical Society Reviews, 2018, 47(3): 715-735.
[30] XU Z, CHEN Y, ZHANG Z et al. Progress of research on underpotential deposition——I. Theory of underpotential deposition[J]. Wuli Huaxue Xuebao/ Acta Physico - Chimica Sinica, 2015, 31(7): 1219-1230.
[31] PANGAROV n. Thermodynamics of electrochemical phase formation and underpotential metal deposition[J]. Electrochimica Acta, 1983, 28(6): 763-775.
[32] KAYASTH S. ELECTRODEPOSITION STUDIES OF RARE EARTHS[J]. Methods in Geochemistry and Geophysics, 1972, 6(C): 5-13.
[33] KONDO T, TAKAKUSAGI S, UOSAKI K. Stability of underpotentially deposited Ag layers on a Au(1 1 1) surface studied by surface X-ray scattering[J]. Electrochemistry Communications, 2009, 11(4): 804-807.
[34] GASPAROTTO L H S, BORISENKO N, BOCCHI N et al. In situ STM investigation of the lithium underpotential deposition on Au(111) in the air- and water-stable ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide[J]. Physical Chemistry Chemical Physics, 2009, 11(47): 11140-11145.
[35] SARABIA F J, CLIMENT V, FELIU J M. Underpotential deposition of Nickel on platinum single crystal electrodes[J]. Journal of Electroanalytical Chemistry, 2018, 819(V): 391-400.
[36] BARD A J, FAULKNER L R, SWAIN E et al. Fundamentals and Applications[M]. John Wiley & Sons, Inc, 2001.
[37] SCHWEINER F, MAIN J, FELDMAIER M et al. Impact of the valence band structure of Cu2O on excitonic spectra[J]. Physical Review B, 2016, 93(19): 1-16.
[38] XIONG L, HUANG S, YANG X et al. P-Type and n-type Cu2O semiconductor thin films: Controllable preparation by simple solvothermal method and photoelectrochemical properties[J]. Electrochimica Acta, 2011, 56(6): 2735-2739.
[39] KAZIMIERCZUK T, FRÖHLICH D, SCHEEL S et al. Giant Rydberg excitons in the copper oxide Cu2O[J]. Nature, 2014, 514(7522): 343-347.
[40] RAEBIGER H, LANY S, ZUNGER A. Origins of the p-type nature and cation deficiency in Cu2 O and related materials[J]. Physical Review B - Condensed Matter and Materials Physics, 2007, 76(4): 1-5.
[41] 舒雲(yun) . Cu2O薄膜的電化學製備及其光電化學性能的研究[D]. 雲(yun) 南大學物理與(yu) 天文學院,2019.
展示全部 